Viagra Plus
Viagra Plus dosages: 400 mg
Viagra Plus packs: 30 pills, 60 pills, 90 pills, 120 pills, 180 pills, 270 pills, 360 pills

Viagra plus 400 mg discount
Such effects could embrace changes in tensile power erectile dysfunction causes mayo buy viagra plus 400 mg on-line, color erectile dysfunction help without pills buy viagra plus 400 mg low price, odour and gasoline formation of polymers. The supplies most affected embrace acetal, fluorinated ethylene propylene, polytetrafluoroethylene and polyvinyl acetate. The complete absorbed power determines the extent of physical and chemical reactions that happen, and so damage is cumulative. For sterilization purposes, exposure instances can be long, but the Electromagnetic radiation radiation outcomes when the nucleus nonetheless has an extreme quantity of power even after the emission of or particles. This power is dissipated in the form of very quick wavelength radiation which, because it has no mass or cost, travels with the speed of light, penetrating even sheets of lead. Although travelling in a wave kind, radiation behaves as whether it is composed of discrete packets of power called quanta (photons). They have properties similar to these of -rays despite originating from a shift in electron energy somewhat than from the nucleus. Units of radioactivity the unit of activity is the becquerel (Bq), which is equal to one nuclear transformation per second. The energy of radiation is measured in electronvolts (eV) or millions of electronvolts (MeV). In this case the ionizing radiation is immediately responsible for the harm by inflicting a direct hit on a sensitive goal molecule. The passage of ionizing radiation via water causes ionization alongside and instantly next to the track and the formation of free radicals and peroxides. These peroxides and free radicals are extremely reactive and destructive and are liable for both the killing capability and the flexibility to modify the properties of polymers. Resistance to radiation is genetically determined, and a particularly resistant bacterium called Deinococcus radiodurans can face up to a radiation dose up to 5000 Gy, in contrast with Escherichia coli, which is killed by 800 Gy. Oxygen has already been talked about as having a major influence on the antimicrobial results of radiation, as elevated levels of hydroperoxyl radicals result in marked increases in kill. The presence of moisture may also affect sensitivity, with dehydration inflicting a rise in resistance owing to an indirect impact on the formation and mobility of free radicals. Freezing increases radiation resistance owing to the reduction of mobility of free radicals in the menstruum, preventing them from diffusing to websites of motion at the cell membrane. A variety of natural supplies present a protecting surroundings for microorganisms, and comparability of radiation resistance is tremendously sophisticated by completely different complexities of the media used. Some naturally occurring supplies, particularly meals, may have a profound protecting impact on contaminant micro organism. A broad number of gaseous agents has been used for his or her antimicrobial properties, and a few of the main ones shall be considered here. Toxicity issues embody burns and blistering if the fabric comes into contact with the skin, whereas inhalation ends in lachrymation, headache, dizziness and vomiting. Great care have to be taken to ensure the removing of residual ethylene oxide from treated merchandise. Explosive mixtures are shaped when ethylene oxide is mixed with air at any focus above 3%, and that is particularly dangerous if the gas mixture is confined. The addition of carbon dioxide or fluorinated hydrocarbons will eliminate this threat, and for sterilization purposes, fuel mixtures of 10% ethylene oxide and 90% carbon dioxide are typically used. Ethylene oxide is extraordinarily effective at killing microorganisms, and its exercise is said to its action as an alkylating agent. Reactive hydrogen atoms on hydroxyl, carboxyl, sulfhydryl and amino teams can all get replaced with hydroxyethyl groups, thereby interfering with a variety of metabolic actions. Ethylene oxide inactivates the complete spectrum of microorganisms, together with endospores and viruses. The difference in resistance between endosporeforming micro organism and vegetative cells is simply of the order of 5 to 10 instances, compared with severalthousand-fold differences with different bodily and chemical processes. In addition, no microorganism of genetically decided excessive resistance has been found. The formation of aggregates of cells will end in these cells on the centre of the mixture surviving an otherwise lethal dose of radiation. Similarly, microorganisms suspended in water face up to significantly larger doses of radiation than within the dry state, owing to lack of penetration of the radiation. Suspension of bacteria in broth containing organic matter such as proteins increases the resistance of the cells nonetheless further. The stage of progress of the tradition will affect the sensitivity of the cells, with most sensitivity being shown during the logarithmic phase. Other factors proven to affect radiation resistance embrace pH, temperature and humidity, though the impact of the last parameter is still considerably confused. Factors affecting the activity of ethylene oxide the bactericidal activity of ethylene oxide is proportional to the partial stress of the fuel in the reaction chamber, the exposure time, the remedy temperature, and level and type of contamination. At room temperature, the time taken to reduce the initial focus of cells by 90% can be very sluggish. For this cause, elevated temperatures of 50 �C to 60�C are really helpful, and these end in significantly elevated rates of kill. Concentrations of ethylene oxide between 500 mg L-1 and 1000 mg L-1 are usually used. Microorganisms could also be protected against the action of ethylene oxide by occlusion within crystalline material or when coated with natural matter or salts. Biological indicators used to test the efficacy of ethylene oxide therapy make use of spores of B. Its bactericidal powers are superior to those of ethylene oxide (concentrations of 3 mg L-1 to 10 mg L-1 are effective) nevertheless it has weak penetrating energy and is actually solely a surface bactericide. It acts as a mutagenic agent and an alkylating agent, reacting with carbonyl, thiol and hydroxyl teams. In order to be efficient, the fuel should dissolve in a film of moisture surrounding the bacteria. Formaldehyde used along side low-temperature steam is a really effective sterilization medium. Peracetic acid the toxic nature of ethylene oxide and formaldehyde has prompted the seek for further gaseous sterilants. Peracetic acid has been extensively used as an aqueous solution, but its use within the gas part is extra limited. The main attraction of hydrogen peroxide as an antimicrobial agent is the truth that its decomposition products are oxygen and water. Most work on the antimicrobial properties of hydrogen peroxide has been carried out on aqueous solutions, the place it has been proven to have a great range of exercise, together with towards bacterial spores. The biocidal efficacy of the vapour section is less than that in answer and is influenced by environmental situations. The vapour, which is extremely irritating to the eyes, nostril and throat, could be generated either from solid polymers corresponding to paraformaldehyde or from an answer of 37% formaldehyde in water (formalin). Propylene oxide Propylene oxide is a liquid (boiling point 34 �C) at room temperature which requires heating to volatilize.
Purchase 400 mg viagra plus amex
From a physical instability perspective impotence natural home remedies viagra plus 400 mg otc, proteins are susceptible to causes of erectile dysfunction in 30s cheap 400 mg viagra plus free shipping denature (alter their native secondary or tertiary structure) on exposure to warmth, extremes of pH or natural solvents. One of an important challenges associated with protein formulation is to forestall protein aggregation in answer. Protein aggregation to kind non-native aggregates, while not fully understood, is thought to be mediated by both hydrophobic attraction between the less polar elements of the protein molecule or covalent bonding between two protein molecules. Partial unfolding to reveal hydrophobic faces has been implicated in protein aggregation, and therefore strategies which stabilize the protein in its native state stop protein aggregation. The administration of non-native aggregates is undesirable, as protein aggregates are recognized, particularly, to provoke an undesirable immune response, as properly as to compromise the efficacy of the drug. When one is optimizing the in vivo efficacy of proteins, the supply of poor in vivo efficacy must first be totally understood. One major supply of poor efficacy is the precise protein/peptide chemical construction. Whilst there was a huge effort aimed at making peptides orally active, there are solely two oral peptide medication in the marketplace, and each are cyclic peptides: ciclosporin and desmopressin, with desmopressin having unnatural amino acids. Cyclisation, unnatural amino acids and/or deamination undoubtedly makes these peptides less susceptible to gut aminopeptidases and carboxypeptidases. Other supply routes which were tried embrace the nasal route, which is helpful experimentally for delivering peptides to the brain and has been used commercially for the systemic (not specifically braintargeted) supply of calcitonin, a 32-amino acid peptide used to deal with osteoporosis. However, the dose volumes through this route are limited to roughly a hundred and fifty �L, and a dose of approximately 25 mg is the higher restrict (see Chapter 38). As an illustrative instance, the antiangiogenic monoclonal antibody bevacizumab is formulated with trehalose (a cryoprotectant and preventer of aggregation), phosphate buffer (for pH maintenance) and polysorbate 20 (for stabilization towards interplay with surfaces, unfolding and aggregation). Protein therapeutics are administered parenterally, normally intravenously, though sometimes subcutaneously or intramuscularly. Other nonparenteral routes, such as the nasal route and the pulmonary route, have additionally been attempted. Following intravenous administration, pharmaceutical proteins are generally rapidly cleared from the blood with brief plasma half-lives (Table forty three. For example, the exercise of pegylated human growth hormone is reduced by 75% in comparison with that of the native hormone however its intravenous half-life is elevated 30-fold from 20 minutes to 10 hours. A additional supply challenge for therapeutic proteins in particular is their immunogenicity, whereby proteins generate neutralizing antibodies, which make subsequent doses of the drug ineffective. Delivery methods Protein stabilization the formulation of proteins and peptides includes prevention of chemical degradation and enhancement of in vivo exercise. To prevent chemical degradation a low pH is preferred (pH 3�6) as this limits the reactivity of nucleophiles. Furthermore, drying, particularly freeze-drying, could additionally be used to stabilize proteins in opposition to quite so much of degradative influences. Whilst intravenous delivery of proteins is the norm, to improve patient adherence a subcutaneous formulation of trastuzumab has been developed during which the monoclonal antibody was formulated with recombinant human hyaluronidase to allow the subcutaneous administration of a relatively giant volume (5 mL). Antibody�drug conjugates Antibodies covalently sure to extremely potent therapeutic active ingredients are a model new class of therapeutic: the antibody�drug conjugate. Peptide delivery Peptides, containing 2�50 amino acid residues, are largely administered parenterally, though there are a quantity of merchandise in the marketplace by which peptides are administered via the nasal route. It is estimated that there are 366 million folks with diabetes worldwide, and all insulin-dependent diabetes sufferers administer their insulin, or their insulin is run, through parenteral routes. Rapidly appearing insulin (Novolog) accommodates a mutation in the B28 proline, which is changed with an aspartic acid residue; this prevents insulin from forming hexamers due to cost repulsion, allowing the monomers to be quickly absorbed. Levemir) is ready by a lower of the solubility of insulin such that insulin forms a depot. In this formulation, insulin is stabilized on porous inhalable 2 �m to three �m fumaryl diketopiperazine crystals, with insulin adsorbed on the surface (Technosphere technology). The insulin particles have a excessive surface area, are deposited in the deep lung and dissolve in the lung, permitting insulin to diffuse throughout the alveolar epithelium. The formulation has a time to maximum plasma concentration (tmax) of quarter-hour, and therapeutic levels are maintained for three hours; therefore the formulation is suitable for controlling postprandial glucose ranges and is superior, on this regard, to subcutaneous insulin, which has tmax of two hours. Long-acting peptides are required in sure illness states, such as for the remedy of prostate most cancers by way of chemical castration. In this therapeutic routine, a luteinizing hormone releasing hormone agonist, goserelin, must be administered for a quantity of weeks to achieve its therapeutic impact. These elevated testosterone levels in the end create a unfavorable feedback loop inside 14�21 days and ultimately diminished ranges of testosterone are achieved, a course of termed chemical castration. The higher stage of the much less soluble lactide in the 3-month depot formulation ensures that matrix erosion/degradation and drug release happen at a slower price. Vaccines Introduction Vaccines are administered prophylactically to patients to protect in opposition to infectious illnesses. Mass immunization programmes firstly of the 20th century coupled with access to clean water and the invention of antibiotics have had essentially the most profound effect on human well being ever witnessed. The innate immune response includes an activation of the immune system, the removal of international cells and proteins and an activation of the adaptive immune response. This early immune response offers a rapid and generic defence towards threats and is important for the initiation of the adaptive and longer-lasting immune response, which leads to immunological memory and to the antigen-specific elimination of the pathogen. The release of costimulatory molecules and cytokines as a half of the innate immune response also contributes to the activation of T cells. T-cell activation is followed by B-cell activation and expansion and the release of antibodies specific for the antigen. The mechanism by which vaccines stimulate an immune response has largely informed the development of vaccine supply systems. The antigen is isolated from the cells by ultracentrifugation and chromatographic means. Delivery points the foremost supply challenge surrounding vaccines is the upkeep of the chilly chain (from manufacture to administration) to forestall antigen degradation and ensure potency. It can additionally be fascinating to prevent unwanted bacterial progress and to guarantee a sufficiently high and prolonged immune response, as this will reduce the number of vaccination occasions. Delivery systems Most multiple-use vaccines contain a preservative such because the mercury compound thiomersal. Vaccines may also comprise antibiotics to forestall unwanted bacterial contamination and stabilizers similar to 2-phenoxy esters. Furthermore, as said already, all vaccines have to be saved in a continuous chilly chain to keep antigen efficiency. This is a requirement, and this need for refrigeration contributes significantly to the costs of vaccine distribution and prevents low-resource communities from accessing vaccines. To circumvent the appreciable expense related to the maintenance of a chilly chain, researchers have prepared vaccine�sugar glasses by which a vaccine is combined with trehalose and sucrose and dried on a membrane to be hydrated when required.
Diseases
- Dennis Fairhurst Moore syndrome
- Halal Setton Wang syndrome
- Holocarboxylase synthetase deficiency
- Apraxia
- Infantile digital fibromatosis
- Deafness, X linked, DFN
Viagra plus 400 mg buy cheap on-line
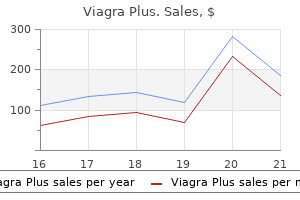
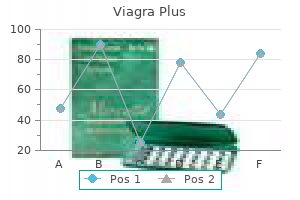
The peak concentration represents the best concentration of the drug achieved in the plasma impotence pump 400 mg viagra plus buy with visa, and is referred to as Cmax erectile dysfunction drug related viagra plus 400 mg cheap otc. This is the time required to achieve the height plasma concentration of the drug after the administration of a single dose. This parameter is related to the rate of absorption of the drug and can be used to assess that rate. Formulation B, which has a slower fee of absorption than formulation A, shows a slower therapeutic onset than formulation A, but its peak plasma concentration lies throughout the therapeutic range. In addition, the period of motion of the therapeutic effect obtained with formulation B is longer than that obtained with formulation A. Hence formulation B seems to be superior to formulation A from a medical viewpoint, in that its peak plasma focus lies within the therapeutic range of the drug and the duration of the therapeutic effect is longer. Formulation C offers a a lot smaller area beneath the plasma concentration�time curve, indicating that a decrease proportion of the dose has been absorbed. This, along with the slower price of absorption from formulation C (the time to peak focus is longer than for formulations A and B), leads to the height plasma concentration not reaching the minimum effective concentration. This simple hypothetical example illustrates how differences in bioavailability exhibited by a given drug from completely different formulations can lead to a affected person being over medicated, undermedicated or accurately medicated. It is essential to notice that the study of bioavailability primarily based on drug focus measurements within the plasma (or urine or saliva) is difficult by the truth that such concentration�time curves are affected by components aside from the biopharmaceutical factors of the drug product itself. It is quite ordinary for different medication to exhibit different charges of absorption, metabolism, excretion and distribution, totally different distribution patterns and differences in their plasma binding phenomena. Therefore it will be extremely tough to attribute variations in the concentration�time curves obtained for various medicine introduced in several formulations to differences in their bioavailabilities. Cumulative urinary drug excretion curves Measurement of the focus of intact drug and/or its metabolite(s) in the urine can be used to assess bioavailability. If an orally administered drug is subject to intestinal metabolism or first-pass liver metabolism, then measurement of the principal metabolite or of intact drug plus metabolites within the urine would give an overestimate of the systemic availability of that drug. It should be remembered that the definition of bioavailability is by method of the extent and the speed at which intact drug seems in the systemic circulation after the administration of a recognized dose. The assessment of bioavailability by urinary excretion relies on the idea that the looks of the drug and/or its metabolites within the urine is a function of the rate and extent of absorption. This assumption is only legitimate when a drug and/or its metabolites are extensively excreted within the urine, and when the rate of urinary excretion is proportional to the concentration of the intact drug in the blood plasma. The essential parameters in urinary excretion studies are the cumulative amount of intact drug and/or metabolites excreted and the speed at which this excretion happens. A cumulative urinary excretion curve is obtained by amassing urine samples (resulting from the whole emptying of the bladder) at known intervals after a single dose of the drug has been administered. Urine samples should be collected till all of the drug and/or its metabolites have been excreted (this is indicated by the cumulative urinary excretion curve turning into parallel to the abscissa) if a comparison of the extent of absorption of a given drug from different formulations or dosage types is to be made. The whole quantity of intact drug (and/or its metabolite(s)) excreted in the urine at point Z corresponds to the time at which the plasma concentration of intact drug is zero and essentially all of the drug has been eradicated from the body. The complete quantity of drug excreted at level Z could additionally be quite different from the total amount of drug administered. Use of urinary drug excretion curves in bioavailability studies In order to illustrate how cumulative urinary excretion curves can be used to compare the bioavailabilities of a given drug from completely different formulations, let us contemplate the urinary excretion data obtained following the administration of single equal doses of the three totally different formulations, A, B and C, of the same drug to the identical wholesome particular person by the identical extravascular route on three different events. The cumulative urinary excretion curves show that the rate at which the drug appeared within the urine. As the entire amount of intact drug excreted is assumed to be associated to the whole quantity absorbed, the cumulative urinary excretion curves for formulations A and B indicate that the extent of drug absorption from these two formulations is similar. Thus both the plasma concentration�time curves and the corresponding cumulative urinary excretion curves for formulations A and B show that the extent of absorption from these formulations is equal, despite the drug being released at different rates from the respective formulations. Consideration of the cumulative urinary excretion curve for formulation C shows not solely that this formulation leads to a slower rate of look of intact drug in the urine but in addition that the whole quantity of drug eventually excreted is much lower than from the opposite two formulations. This is as a outcome of when a drug is delivered intravenously, the whole administered dose is introduced immediately into the systemic circulation, i. The absolute bioavailability of a given drug using plasma data may be calculated by comparing the whole areas under the plasma concentration�time curves obtained following the administration of equivalent doses of the drug through any route of administration and following delivery by way of the intravenous route in the identical particular person on totally different occasions. Absolute and relative bioavailability Absolute bioavailability the absolute bioavailability of a given drug from a dosage form is the fraction (or percentage) of the administered dose which is absorbed intact into the systemic circulation. Absolute bioavailability may be calculated by comparability of the total amount of intact drug that reaches the systemic circulation after the administration of a known dose of the dosage kind via a route of administration with the total amount that reaches the systemic circulation after the administration of an equivalent dose of the drug in the form of an intravenous bolus injection. Often the dose administered intravenously is decrease to avoid poisonous unwanted side effects and for ease of formulation. Care should be taken when completely different doses are used to calculate bioavailability knowledge as generally the pharmacokinetics of a drug are nonlinear and different doses will then result in an incorrect figure for absolutely the bioavailability if it is calculated using a easy ratio, as in Eq. The absolute bioavailability based on urinary excretion data could also be determined by evaluating the entire cumulative quantities of unchanged drug ultimately excreted in the urine following administration of the drug through an absorption site and the intravenous route (bolus injection) on totally different occasions to the identical particular person. For equivalent doses of administered drug, absolute bioavilability = (X u)abs (X u)iv (21. If different doses of the drug are administered, absolute bioavilability = (X u)abs Dabs (X u)iv Div (21. It should be famous that the worth calculated for the absolute bioavailability will solely be legitimate for the drug being examined if the kinetics of distribution and elimination are independent of the route and time of administration and the size of dose administered (if different doses are administered by the intravenous route and absorption site). The latter is both an orally administered resolution (from which the drug is known to be properly absorbed) or an established industrial preparation of confirmed scientific effectiveness. As for absolute bioavailability, relative bioavailability may be expressed as a fraction or as a share. Urinary excretion data may also be used to measure relative bioavailability as follows: relative bioavilability = (X u)test (X u)standard (21. If completely different doses of the test and normal dosage varieties are administered on separate events, the whole quantities of unchanged drug in the end excreted within the urine per unit dose of the drug should be used on this equation. It ought to be famous that measurements of relative and absolute bioavailability primarily based on urinary excretion information can also be made in phrases of both the whole quantity of the principal drug metabolite or the total amount of unchanged drug plus its metabolites ultimately excreted within the urine. However, the assessment of relative and absolute bioavailability by means of urinary excretion knowledge is based on the assumption that the whole amount of unchanged drug (and/or its metabolites) finally excreted in the urine is a mirrored image of the total quantity of intact drug getting into the systemic circulation (as mentioned for cumulative urinary excretion curves earlier). Relative bioavailability measurements are often used to decide the effects of dosage kind variations on the systemic bioavailability of a given drug. A more detailed account of the affect of these components on bioavailability is given in Chapter 20. Bioequivalence Bioequivalence is the term used to describe the biological equivalence of two preparations of the identical drug. This definition of bioequivalence accommodates two product sorts, pharmaceutical equivalents and pharmaceutical alternatives. Pharmaceutical equivalents means the drug merchandise are in identical dosage forms that contain equivalent amounts of the equivalent lively drug i.
Generic viagra plus 400 mg
Detailed sampling and testing procedures are given in pharmacopoeias erectile dysfunction medication online viagra plus 400 mg on line, and further details can be present in Chapter 14 erectile dysfunction and diabetes treatment viagra plus 400 mg buy discount line. For terminally sterilized merchandise, biologically based and automatically documented bodily proofs that present correct therapy during sterilization present greater assurance than the sterility check. This technique of assuring sterility is termed parametric launch and is outlined as the release of a sterile product based mostly on course of compliance with physical specs. Parametric release is appropriate for all absolutely validated terminal sterilization processes really helpful by the European Pharmacopoeia. The process of validation requires that the suitable documentation is obtained to present that a process is constantly complying with predetermined specifications. For the validation of sterilization processes, two types of knowledge are required: commissioning information and efficiency qualification information (Box 17. Commissioning knowledge refer mainly to the set up and characteristics of the equipment, and the performance knowledge ensure that the gear will produce the required sterility assurance level. The efficiency qualification data can be divided into bodily and organic performance knowledge (Box 17. The use of organic indicators (discussed in the next section) requires good information of the inactivation kinetics. Performance qualification knowledge should be reevaluated following a change to the preparation or product and its packaging, the loading sample or the sterilization cycle. Routine tests 290 are carried out to demonstrate that every one parts of the sterilizer have been correctly installed (installation qualification) and that they operate properly, with sterilizing conditions reaching each a part of the load (operation qualification; McDonnell, 2007). The test strategies used range according to the sterilization technique and should contain the use of physical indicators, chemical indicators and organic indicators. Sensors upkeep and calibration are essential to ensure the validity of parametric release (Berube et al. Another example is a Temptube, which is a glass tube containing a chemical with a selected melting level indicated by a color change. They must be used in the first cycle of the day as an gear perform check (McDonnell, 2007). The standardized test pack is placed in the centre of porous load sterilizers, and if the method is right. The efficiency of chemical indicators may be altered by the storage situations earlier than and after use and by the method used. Biological indicators include a service or package containing a standardized preparation of outlined microorganisms of recognized resistance to a selected mode of sterilization (Berube et al. The carriers used are often manufactured from filter paper, a glass slide, chrome steel or a plastic tube. The service is roofed to stop deterioration or contamination while still allowing entry of the sterilizing agent (British Pharmacopoeia Commission, 2017a). After publicity to the sterilization course of, the indicators are eliminated aseptically and incubated in appropriate media to detect the presence of surviving microorganisms. If no development happens, the sterilization process is alleged to have had enough lethality (Berube et al. Testing filtration efficacy Compared with other sterilization strategies, the potential threat of failure is higher for filtration sterilization. This means that it might be advisable to add an additional prefiltration stage using a bacteria-retentive filter. Confidence within the filters used is of prime importance throughout filtration sterilization. Each batch of filters is examined to be positive that they meet the specifications for launch of particulate materials, mechanical power, chemical characteristics. The microbial problem test is used to reveal that a filter is capable of retaining microorganisms. This is generally carried out with a suspension of no much less than 107 colony-forming models (see Chapter 14) of Pseudomonas diminuta per square centimetre of active filter surface. After filtration of a bacterial suspension prepared in tryptone soya broth, the filtrate is collected and incubated at 32 �C. Integrity tests are used to confirm the integrity of an assembled sterilizing filter earlier than use and to confirm integrity after use. The exams used have to be acceptable to the filter type and the stage of testing and should include bubble point checks, strain maintain exams and diffusion rate tests. The bubble point test is the oldest and one of the most extensively used nondestructive exams. It measures the stress (bubblepoint pressure) wanted to pass gasoline through the most important pore of a wetted filter. In apply, the strain required to produce a gradual stream of gasoline bubbles via a wetted filter is usually used because the bubble level. The basis of the take a look at relies on the holes through the filter resembling uniform capillaries passing from one facet to another. They measure the rate of circulate of a gasoline as it diffuses by way of the water in a wetted filter. Commercial products such as ProReveal can produce a high degree of confidence within the effectiveness of washer disinfector processes. Limitations of sterilization methods Sterilization processes can contain some excessive conditions, similar to excessive temperatures, high strain, a vacuum and pressure pulsing, or the usage of toxic substances, which might injury the product and/or its packaging. The alteration of a pharmaceutical preparation may result in reduced therapeutic efficacy or affected person acceptability, and harm to the container might result in the poststerilization contamination of the product. There needs to be a balance between acceptable sterility assurance and acceptable damage to the product and container. Knowledge of the preparation and packaging design, and the choice and understanding of the sterilization applied sciences help in making the appropriate selection to achieve maximum microbial kill while reducing the risk of product and packaging deterioration. Limitations related to established and recommended procedures are often linked to the nature of the process. Summary the achievement of sterility is a complex course of that requires correct documentation. Therefore the sterility of a product has to be assured by the applying of an acceptable validation course of. It is necessary that the sterilization methodology is appropriate with the preparation or product, including its last container or packaging, and combines effectiveness and the absence of detrimental results. Although not described in detail in this chapter, the choice of the container/packaging must enable the optimum sterilization to be utilized and guarantee that sterility is maintained after the process. In particular, the microbiological high quality of ingredients for pharmaceutical preparations and the removal/reduction of bioburden must be monitored. Monitoring the critical parameters of the sterilization course of will make sure that the predetermined conditions (during validation) are met.
Viagra plus 400 mg buy on line
By spray-drying a suspension of drug particles in a liquid erectile dysfunction when cheating viagra plus 400 mg purchase amex, which can include a dissolved binder erectile dysfunction drugs research viagra plus 400 mg buy generic, comparatively small spherical granules with uniform dimension could be prepared. The process is of restricted use, aside from the preparation of fillers or diluents for direct compaction. The granules can present good compactability, and this presents the possibility of granulating a drug suspension without a separate drying step for the drug substance. The formation of granules by compacting the powder into large compacts which are subsequently comminuted into smaller granules is an alternate approach to granulation. The method can be used as a way of avoiding publicity of the powder to moisture and heat and is also referred to as dry granulation. In addition, for powders of very low bulk density, compaction could be an efficient means to enhance markedly their bulk density. Depending on the supposed major perform, the excipients to be used in tablets are subcategorized into totally different groups. However, one excipient can have an result on the properties of a powder or the tablet in numerous ways, and tons of substances used in pill formulations can thus be described as multifunctional. The capabilities of the most common kinds of excipients utilized in tablets are described within the following sections. Disintegrant Solution binder Dry binder Glidant Filler (or diluent) In order to form tablets of a measurement appropriate for dealing with, a lower restrict by means of powder volume and weight is required. Therefore a low dose of a potent drug requires the incorporation of a substance into the formulation to increase the bulk quantity of the powder and therefore the dimensions of the pill. The best filler should fulfil a sequence of requirements, corresponding to: Lubricant Antiadherent � � � � be chemically inert; be nonhygroscopic; be biocompatible; possess good biopharmaceutical properties. Crystalline lactose is shaped by precipitation and, depending on the crystallization conditions, -monohydrate or -lactose (an anhydrous form) may be formed. By thermal therapy of the monohydrate form, crystalline -anhydrous particles may be ready. Depending on the crystallization circumstances and the use of subsequent size discount by milling, lactose of different particle sizes is obtained. Amorphous lactose can be prepared by the spraydrying of a lactose answer (giving nearly utterly amorphous particles) or a suspension of crystalline lactose particles in a lactose solution (giving aggregates of crystalline and amorphous lactose). Amorphous lactose dissolves extra quickly than crystalline lactose and has better compactability. Other sugars or sugar alcohols, such as glucose, sucrose, sorbitol and mannitol, have been used as alternative fillers to lactose, primarily in lozenges or chewable tablets, because of their nice taste. Mannitol has a negative warmth of solution and imparts a cooling sensation when sucked or chewed. Apart from the sugars, maybe essentially the most widely used fillers are powdered celluloses of different sorts. Celluloses are biocompatible, are chemically inert and have good tablet-forming and disintegrating properties. They are suitable with many medication however, owing to their hygroscopicity, could also be incompatible with medicine vulnerable to chemical degradation within the strong state. The most common kind of cellulose powder used in pill formulation is microcrystalline cellulose. The name indicates that the particles have both crystalline and amorphous areas, depending on the relative place of the cellulose chains within the stable. The diploma of crystallinity might differ relying on the supply of the cellulose and the preparation procedure. The diploma of crystallinity will have an effect on the bodily and technical properties of the particles. Microcrystalline cellulose is ready by hydrolysis of cellulose followed by spray-drying. Depending on the preparation situations, aggregates of various particle measurement may be prepared which have different flowabilities. A last necessary example of a typical filler is an inorganic substance, dicalcium phosphate dihydrate. The substance could be obtained in both a nice particulate form, primarily used in granulation, and an aggregated type. The latter possesses good flowability and is used in tablet production by direct compaction. Dicalcium phosphate dihydrate is slightly alkaline and may thus be incompatible with medication sensitive to alkaline situations. Matrix former In order to affect or management the release of the drug from the pill, i. The matrix former is often a polymer or a lipid and may constitute a significant fraction of the total tablet weight. When the objective is to increase drug dissolution, the matrix former is often a water-soluble substance or a lipid, and the drug is dissolved or suspended as fine particles within the matrix. When the target is to extend the drug release, the matrix former could be both an insoluble substance (a polymer or a lipid) or a substance that forms a gel in touch with water. The drug is normally dispersed in particulate kind within the matrix (for more details, see later). Disintegrant A disintegrant is included within the formulation to ensure that the tablet, when in touch with a liquid, breaks up into small fragments, which promotes fast drug dissolution. Ideally, the tablet ought to break up into individual drug particles so as to produce the most important potential effective floor area during dissolution. Deaggregation into drug particles will arrange circumstances for the fastest attainable dissolution of the drug. The second step, granule disintegration or deaggregation, could end result in the formation of smaller aggregates or, if pushed to an ideal finish point, discrete drug particles. The end level of the second step will probably depend upon a sequence 528 of things, such because the relative solubility and particle size of the drug and excipient particles of the granule. For example, in a situation where the drug particles are of low or very low solubility and are mixed with a hydrophilic filler and binder of excessive solubility, the excipients might dissolve rapidly, resulting in a dispersion of fantastic drug particles. During formation of a pill, the unique drug particles might fracture, and the resultant measurement distribution of the drug particles could thus not necessarily equate to that of the original particles. Furthermore, the drug particles may also deform plastically throughout compression, which in addition might change their form and look (see additionally later). However, as two major processes are concerned in the disintegration occasion, the disintegrants to be utilized in plain tablets are categorised here into two varieties: 1. These disintegrants act by facilitating the transport of liquids into the pores of the pill, with the consequence that the tablet may break up into fragments. One obvious sort of substance that may promote liquid penetration is surface-active agents. Such substances are used to make the drug particle surfaces extra hydrophilic and thus promote the wetting of the solid and the penetration of the liquid into the pores of the tablet.
400 mg viagra plus with amex
Secretory IgA can additionally be more cross-reactive than IgG and might provide safety towards different strains of the virus erectile dysfunction at the age of 21 cheap viagra plus 400 mg without prescription. Nevertheless impotence natural treatment clary sage 400 mg viagra plus visa, as a outcome of the nasal mucosa is exposed to a multitude of antigens current in the environment, tolerance mechanisms limit the resultant immune response. The benefits of nasal vaccination when compared with needle-based supply techniques embody a lowered threat of needle-stick accidents and risk of infection from the reuse of needles, elevated affected person adherence among sufferers with needle phobia, a decreased need for vaccines to be administered by educated health care professionals and presumably a decreased want for chilly chain storage and distribution, if vaccines can be formulated as dry powders. In addition, use of the route provides a ready means of vaccinating massive inhabitants teams. In contrast to the leaky barrier introduced by the endothelial cells of the capillaries in the peripheral circulation, the endothelial cells within the brain exhibit low rates of pinocytosis and are joined by tight junctions which limit the paracellular diffusion of hydrophilic solutes from the blood into the mind. This is at present an area of great research interest, and studies have proven that each low molecular weight drugs and excessive molecular weight peptides and proteins appear to have the power to access the brain following intranasal delivery. It is possible that a drug will gain entry to the brain following its absorption into the systemic circulation and never via a direct route, and it may be very important get rid of or account for this possibility within the design of research looking for to establish or quantify direct nose-to-brain transport. It also needs to be famous that most of the studies of direct nose-to-brain transport have been undertaken in rats, which differ in their nasal anatomy in contrast with people; the nasal passages of the rat have the next surface area to volume ratio than those of humans (51. Another pathway includes substances crossing the other cells of the olfactory epithelium. Tight junctions exist between cells within the olfactory epithelium, however the common turnover of cells in the epithelium may lead to loosening of the tight junctions, helping the paracellular transport of higher molecular weight substances. Immunohistochemical studies have found the efflux transporter P-gp localized to the endothelial cells lining the olfactory bulb and the olfactory epithelium. Because drugs must be contained in the epithelial cell to work together with the binding website of P-gp, this can mainly affect those drugs crossing the supporting cells of the olfactory epithelium through the transcellular route. The trigeminal nerve, which innervates the respiratory epithelium of the nasal cavity, additionally feeds into numerous areas of the brain and will probably be exploited for nose-to-brain drug delivery. One major problem is the inaccessibility of the olfactory area of the nasal cavity coupled with the poor permeation capacity of sure forms of molecule (including peptides and proteins) across the olfactory epithelium. There is a necessity for a formulation containing a suitable nasal permeation enhancer and a bioadhesive materials which may be delivered from a nasal gadget that is in a position to goal the formulation to the olfactory area. Nasal supply methods Nasally administered medicines could be formulated as ointments or lotions but most usually as a liquid (solution, gel or suspension) or as a powdered strong (Tables 38. The formulation issues with every of those dosage forms have been thought of in Chapters 24, 26, 27 and 28. As a consequence, multidose liquid dosage forms can require the inclusion of antimicrobial preservatives to prevent the expansion of contaminating microorganisms. There is proof that some of these preservatives can irritate the nasal mucosa and/or injury the cilia and subsequently compromise mucociliary clearance, particularly if used over a long period. The strategies to minimize or keep away from such effects include the alternate use of the nostrils, if long-term day by day dosage is required, and using pressurized containers or unit-dose supply techniques (Table 38. There is a move towards delivery systems that deliver an correct metered dose and away from dosage varieties similar to nasal drops, which require considerable skill, dexterity and even flexibility (in terms of mobility) to be applied uniformly across the mucosa. Smaller volumes (<100 �L) are inclined to persist longer than bigger volumes, which can drip from the nostril after supply. Powdered solids are inclined to stay within the nasal cavity for longer than liquids, as a preliminary hydration step generally occurs before mucociliary clearance reaches maximal efficiency. This can delay the time over which systemic drug absorption can occur or the duration of motion of a locally appearing drug. Messy to apply Uncontrolled dose (only for medicine with large therapeutic window and excessive tolerability) For medication with localized effects solely Often used for nasal decongestants. Uncooperative sufferers can expel most of the dose by blowing via the nostril Volume administered is subject to the strategy of the user and this form is appropriate only for medication with a big therapeutic window Spray nozzle (either metered-dose pump or syringe) produces fantastic droplets (usually 25 �m to 50 �m). Available as unit dose or reservoir (multiple doses) (generally three mL to 50 mL) spray bottle. A calibrated tube for paediatric and grownup use; involves one finish of a tube containing the dose being positioned within the nostril and the other within the mouth. A tight-fitting nozzle is placed in a single nostril and a metered dose (dispensed from a reservoir) is aerosolized by the patient blowing via a mouthpiece. The nasal cavity is sealed by this action, preventing drug loss to the mouth and throat, and droplets are carried beyond the nasal valve and unfold all through the cavity. Clinical trials have been carried out for therapy of migraine, nasal polyps, sinusitis and autism For instance, droplet size may be controlled (generally eight �m to 30 �m) to maximize nasal deposition. Dry powder formulations tend to adhere better to the nasal mucosa, permitting a longer time for absorption. Control of particle measurement is necessary as a outcome of particles smaller than 10 �m can move past the nasal turbinates towards the lung, whereas particles larger than 50 �m could be cleared more quickly by mucociliary clearance and nostril blowing. An attention-grabbing advance in nasal supply devices which shows helpful potential in delivering nasal formulations entails breath-actuated bidirectional delivery (Table 38. The system is constructed such that the aerosolization of the powder is initiated by patients themselves exhaling by way of the mouth against a resistance. This motion closes the taste bud and separates the nasal cavity from the oral cavity. A communication pathway remains between the two nostrils, situated behind the nasal septum. The expired air (and aerosolized powder) blown into one nostril is turned by way of 180�, passes via the pathway and leaves through the second nostril, guaranteeing that powder deposits all through the cavity. Summary the potential of delivering drugs and vaccines to the physique through the nasal cavity is way from being absolutely realized. There are active programmes inside numerous pharmaceutical and biotechnological companies seeking to use the route. It is restricted by method of the dose that can be delivered, both on account of solubility of the drug (given the volume of liquid that can be delivered comfortably) or in terms of the quantity of powder or semisolid that can be tolerated per dose. Once drug is delivered, it should be absorbed quickly otherwise the conventional clearance mechanisms will take away it from the absorbing epithelium and end in decreased bioavailability. Strategies are subsequently being used to both prolong the absorption window (through the use of mucoadhesive excipients) or enhance permeability (by numerous means, including using permeation enhancers). As with pulmonary delivery nevertheless, the primary packaging is an important part of the final drugs, as this additionally forms a part of the delivery gadget. There is much scope to develop novel units with improved dose allotting and superior concentrating on (perhaps to the olfactory region) or more efficient coating of mucosa. Drug growth programmes need to contemplate each the gadget and the formulation as a whole, considering the therapeutic function of the drugs. Nasal drug supply units: characteristics and performance in a clinical perspective-a evaluate. The nasal 688 method to delivering treatment for mind ailments: an anatomic, physiologic, and supply know-how overview. Intranasal delivery of systemic-acting medicine: small-molecules and biomacromolecules. Drug delivery to the attention is considered one of the most necessary areas of contemporary ocular therapy and presents many opportunities and challenges. The current market for ophthalmic prescribed drugs is price many billions of dollars a 12 months. The entrance of the eye is accessible, and conditions affecting it can be handled by simple topical eye drops.
Myroxylan toluiferum (Tolu Balsam). Viagra Plus.
- Bedsores, bronchitis, cancer, cough, cracked nipples, lips, reducing lung swelling (inflammation), and minor skin cuts.
- Are there safety concerns?
- How does Tolu Balsam work?
- Dosing considerations for Tolu Balsam.
- What is Tolu Balsam?
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96373
Viagra plus 400 mg generic with mastercard
Povidone was used prior to now for this purpose erectile dysfunction pump demonstration 400 mg viagra plus buy free shipping, but safety considerations about this compound when injected intramuscularly have led to it falling out of favour erectile dysfunction quran 400 mg viagra plus purchase visa. A suitable nonionic injectable surfactant such as a polysorbate may be included in a suspension formulation to help the uniform dispersion of the suspended drug substance. This latter methodology of aseptic manufacturing clearly carries the chance of microbial contamination of the product in the course of the filling and sealing process, therefore the choice for sterilization of the drug product contained in the sealed container after filling (terminal sterilization). Ampoules Small-volume parenteral products are often packaged in glass or plastic ampoules. The use of glass and plastics as packaging supplies for pharmaceutical products is discussed in Chapter forty six. Glass ampoules range in size sometimes from 1 mL to 10 mL in volume, though larger sizes are available. The glass chosen is referred to as sort I or borosilicate glass, which is less alkaline than the glass often used for beverages and other purposes. Because of the baking course of required to fuse the ceramic to the glass, this acts as a weak point at which the ampoule could be easily snapped open by hand. The main disadvantages of glass ampoules are the fragility of the container, the potential for deposition of glass particles into the drug product on opening and the potential for injury to the fingers of the person opening the ampoule. The problem of fragility is overcome by use of sturdy Thus you require the addition of three 93% w/v anhydrous glucose (i e 3 93 g anhydrous glucose per a hundred mL) to make the potassium chloride resolution isotonic with plasma earlier than use. This is especially necessary for injections supplied as powders for reconstitution, because the well being care practitioner needs to have the ability to see that the drug substance has completely dissolved in the diluent before withdrawal of the dose. Large-volume infusion fluids typically produce other medication added to them, so once more the readability of the container is important to enable the right mixing of the product to be assessed and to check that large particles. Whatever type of container is used, it have to be tightly sealed to maintain the sterility of the injection before use and to stop other contaminants. In desire, parenteral merchandise are manufactured and added to the primary container, which is then sealed and the product is then terminally sterilized inside the container, for instance by use of moist warmth in an autoclave (see Chapters sixteen and 17). Glass particles can be removed from the product by the contents of the ampoule being drawn by way of a filter straw or quill right into a syringe. The advantages of glass ampoules are low price and (if kind I glass is used) little or no interaction between the container and the product. Plastic ampoules are ready by a extremely automated blow�fill�seal product process. The filling machine is loaded with the drug answer and with plastic granules (polyethylene and/or polypropylene), that are then melted. The molten plastic is blown into the ampoule-shaped mould of the machine to type the physique of the ampoule, the body of the ampoule is filled with product and then the lid of the ampoule is moulded onto the top of the ampoule to kind a seal. The sealed ampoule is opened by the lid being twisted off, and only a few particles are generated to contaminate the product. The disadvantages are that it is a more costly course of, and is suitable just for drug products formulated as easy solutions. A full comparison of glass and plastics as supplies for pharmaceutical packaging is provided in Chapter forty six. To withdraw a dose from a vial, the cap is eliminated and the septum disinfected with a sterile alcohol wipe. A syringe and needle is used to puncture the rubber closure and take away the required quantity of product. The rubber septum is self-sealing to a excessive diploma, and so more than one withdrawal may be produced from a vial. Products packaged in vials for multiple use will therefore incorporate a preservative to prevent any Vials Vials are containers normally manufactured from type I glass with a reusable synthetic rubber closure. Vials have advantages as containers as they allow multiple withdrawals and are made in sizes often ranging from 5 mL to 100 mL. Vials are sealed with a bromobutyl or chlorobutyl synthetic rubber (elastomer) closure held in place by an aluminium seal crimped around the neck of the glass vial. Synthetic rubber can be latex-free, which is important as sensitization to latex is an rising downside for health care workers. The major drawback is that puncturing the rubber closure can cause large rubber particles to be introduced into the drug product. Infusion bags and bottles Large-volume parenteral merchandise are packaged in glass bottles, collapsible plastic luggage and semirigid plastic bottles, although the use of glass bottles for large-volume parenteral products is turning into a lot much less commonplace. Collapsible baggage normally have an additive port to allow different injectable drugs to be added to the infusion fluid. Additionally, parts can leach out of the plastic, corresponding to monomers and phthalate plasticizers, which may be toxic on longterm exposure. These containers might have an additive port to enable different medication to be added to them. Large-volume glass bottles are essentially the same as glass vials however on a bigger scale. Nowadays, parenteral merchandise may be packaged in syringes, and may thus be presented to the health care skilled or affected person in a ready-to-use format. Such infusion gadgets are used to administer medication intravenously over a protracted interval. They are used for the administration of analgesia postoperatively (which the patient could control on demand) or as part of palliative care. Infusion units can be battery-powered pumps which infuse medication from an attached plastic reservoir. When stuffed, the balloon is tremendously expanded and the drug resolution inside is under strain. The advantage of elastomeric units is that the mechanical strain from the inflated reservoir powers the gadget and no batteries are required. The drawback is that the move price via the restriction valve is temperature delicate, so could be altered depending on how the system is worn by the patient. If the restriction valve is placed next to the pores and skin of the patient, a better than anticipated circulate fee is seen. The administration of a drug at its web site of action can lead to a fast onset of exercise, which can be highly desirable, for instance when bronchodilating medicine for the treatment of asthma are being delivered. The avoidance of first-pass (presystemic) metabolism within the liver may also be advantageous, though the lung itself has some metabolic functionality. The lung can also be used as a route for delivering medication having systemic exercise, due to its giant surface space, the abundance of capillaries and the thinness of the air�blood barrier. This has been exploited in the remedy of migraine with ergotamine and diabetes with insulin, and the potential for delivering biopharmaceuticals, similar to insulin, vaccines and development hormone, via the airways is now well established. The construction of the airways additionally effectively prevents the entry and promotes the removal of airborne overseas particles, including microorganisms. The higher respiratory tract contains the nose, throat, pharynx and larynx; the decrease respiratory tract comprises the trachea, bronchi, bronchioles and the alveolar areas. Simplistically, the airways can be described by a symmetrical model during which each airway divides into two equivalent branches or generations. In reality, the trachea (generation 0) branches into two major bronchi (generation 1), of which the proper bronchus is wider and leaves the trachea at a smaller angle than the left bronchus, and therefore is more prone to receive inhaled material. These divide to produce respiratory bronchioles, which connect with alveolar ducts resulting in the alveolar sacs (generation 23).
Generic 400 mg viagra plus otc
This makes it essential in some circumstances to administer cytotoxic agents repeatedly following surgery for the prevention of scarring impotence due to diabetic peripheral neuropathy 400 mg viagra plus order overnight delivery. The capability of a drug to permeate by way of the sclera is inversely proportional to the molecular size erectile dysfunction surgical treatment options buy discount viagra plus 400 mg on-line. Although no clear correlation exists between drug lipophilicity and the steady-state permeation coefficient for the sclera, medicine with larger lipophilicities exhibit stronger binding to the sclera and longer transport lag times. Injection through the sub-Tenon route is used to administer local anaesthetics, corticosteroids and anticancer brokers. Intravitreal pharmacokinetics Drugs administered into the vitreous humour could be cleared by two routes: the anterior and posterior routes. The physicochemical properties that affect drug clearance are the molecular weight, compound lipophilicity (measured by log P or log D) and dose number (dose/solubility at pH 7. The logarithm of the molecular weight has been found to correlate positively with the vitreal half-life of molecules. This can be defined by the slow diffusion of excessive molecular weight compounds within the vitreous gel, in addition to the remark that prime molecular weight compounds are predominantly eradicated by way of the longer anterior pathway. It has been proposed that hydrophilic molecules are predominantly eliminated by the anterior route. The posterior route is the principle elimination pathway for lipophilic drugs and offers a big floor area and lively transporter mechanisms, thus providing a faster route of elimination than the anterior route. Soluble, low molecular weight drugs may have a vitreous half-life of several hours on intravitreal injection. In contrast, charged excessive molecular weight medication such as the antibody-based therapeutic proteins display a longer vitreous half-life in the vary of 3�7 days as they diffuse more slowly within the vitreous humour than do low molecular weight molecules. Traditional in vitro fashions of ocular pharmacokinetics have been relatively simple, together with easy check tube launch. In vivo fashions can provide pharmacokinetic information, including in people, for dosing with topical or systemic antibiotics earlier than cataract surgical procedure, when aqueous levels may be sampled. New in vitro fashions which mimic the human eye and produce a much more clinically related longer-term result are currently being developed. These deposits have been observed in sufferers months and even a couple of years after the last administration of a triamcinolone injection. It is speculated that these insoluble deposits come up from aggregation or clumping of drug particles. It may even be that a polymorphic conversion of the drug happens within the ocular fluids, leading to a particularly steady, and therefore insoluble, type of triamcinolone which persists within the posterior segment of the attention. Countless numbers of intraocular implants have been shown to carry out successfully in vitro throughout preclinical development; nonetheless, very few carry out satisfactorily in vivo and make it to the clinic. One of the primary causes for this failure is nonbiocompatibility, which triggers the formation of a thick fibrotic capsule around implants, which is commonly referred to as a international physique response. The fibrotic encapsulating tissue is an amalgamation of cells, fibrinogen, fibrin, collagen fibres and different proteins. It is predominantly an inflammatory response, orchestrated by interleukins and reworking progress elements synthesized by epithelial cells. This collagenous fibrotic tissue creates a diffusion barrier for drug molecules and retards biodegradation of the implant. Foreign body reactions are largely influenced by the surface properties of the implant, including contact angle, surface useful groups, water�polymer interactions, roughness, morphology, porosity and get in touch with duration. Repeated intravitreal injections are associated with an elevated risk of scleral injury, poisonous effects on the ocular tissue because of high peak drug concentrations, ocular infection, improve in intraocular strain, incision-related subconjunctival and intravitreal haemorrhage and discomfort related to foreign body sensation and ache within the affected person. A thorough characterization of the compatibility of excipients within the formulation with the lively compound is absolutely needed. Benzalkonium chloride is cationic and therefore is incompatible with anionic medication. Its exercise, and consequently preservative efficacy, is lowered in the presence of multivalent metal ions and anionic and nonionic surfactants. Despite these interactions of benzalkonium chloride with medicine and different excipients, it may nonetheless be essential to use it in some circumstances. Examples of those embody sodium cromoglicate and nedocromil sodium, which kind insoluble emulsion complexes with benzalkonium chloride by way of ion pairing. These insoluble complexes are, nonetheless, removed by filtration in the course of the manufacturing process. Patient adherence and instillation of eye drops the quick half-life of drugs in the anterior chamber requires the frequent administration of eye drops. Studies have shown that almost 50% of glaucoma patients have been nonadherent with respect to their medicine use for greater than 75% of the time. Eye drops are challenging to administer and require coordination, handbook dexterity and vision, necessitating clear directions and patient counselling. Administration of eye drops requires relatively acceptable imaginative and prescient and the power to open and squeeze the bottle. Elderly patients typically suffer from joint illness corresponding to arthritis and can also have poor coordination, which may end up in the drop lacking the eye or the tip of the bottle scratching the cornea. Most glaucoma patients are older individuals, and a study has shown that 17% of glaucoma sufferers depend on others to administer their eye drops. The best way to administer the drop efficiently is to pull the decrease eyelid downwards and administer the drop into the decrease ocular cul-de-sac. Several units can be found for use at the side of totally different eye drop bottles to make this process easier. Dose timing and frequency have also been strongly associated with nonadherence to eye drop remedy. Patients with a 3 times day by day regimen were extra more doubtless to expertise missed doses and likewise had irregular timing of doses in contrast with sufferers with twice every day regimens. This illustrates the significance of designing ophthalmic preparations that present a sustained drug launch and which require much less frequent dosing. Interfacial interactions between transmembrane ocular mucins and adhesive polymers and dendrimers analyzed by surface plasmon resonance. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Current and future ophthalmic drug supply systems: a shift to the posterior phase. Influence of molecular weight and formulation pH on the precorneal clearance fee of hyaluronic acid within the rabbit eye. Reliability of drop size from multi-dose eye drop bottles: is it cause for concern Drug transport in corneal epithelium and blood-retinal barrier: emerging function of transporters in ocular pharmacokinetics. More than 2000 years in the past, Greek physicians used formulations containing salt, vinegar, honey and resins to treat pores and skin lesions and ulcers.
Order 400 mg viagra plus fast delivery
A concentrated answer of gelatin erectile dysfunction 27 viagra plus 400 mg cheap line, 35% to 40% erectile dysfunction more causes risk factors buy viagra plus 400 mg low cost, is prepared using demineralized hot water, 60 �C to 70 �C, in jacketed stress vessels. This is stirred till the gelatin has dissolved, after which a vacuum is applied to take away any entrapped air bubbles. Aliquots of this resolution are allotted into appropriate containers, and the required quantities of dye options and pigment suspensions are added. The viscosity is measured and adjusted to a target value by the addition of sizzling water. This latter parameter is used to management the thickness of capsule shells throughout production: the upper the viscosity, the thicker the shell wall produced. The ready mixes 599 Colourants the colourants that can be used are of two sorts: water-soluble dyes or insoluble pigments. To make a range of colors, dyes and pigments are combined collectively as solutions or suspensions. The dyes used are mostly synthetic in origin and could be subdivided into the azo dyes (those that have an �N=N� linkage) and the non-azo dyes, which come from quite so much of chemical courses. Most dyes used at present are non-azo dyes, and the three most generally used are erythrosine (E127), indigo carmine (E132) and quinoline yellow (E104). Two types of pigment are used: black, purple and yellow iron oxides (E172) and titanium dioxide (E171), which is white and is used to make the capsule opaque. Hypromellose options can be converted right into a gelling system by the addition of a gelling agent, similar to carrageenan, and a co-gelling agent, corresponding to potassium chloride, and used to manufacture capsules on standard unmodified machines utilizing the identical situations as for gelatin. Hypromellose capsules may additionally be made by the new mould dip course of, which depends on the property that the viscosity of solutions increases with temperature. They consist of two elements, which are mirror pictures of each other: on one half the capsule cap is made and on the opposite the capsule physique is made. The machines are housed in massive rooms, the place the humidity and temperature are closely controlled. This holds a set amount of gelatin at a continuing temperature, between forty five �C and 55 �C. The stage of solution is maintained routinely by a feed from the holding hopper. Capsules are shaped by the dipping of sets of moulds, which are at room temperature, 22 �C, into this solution. The moulds are slowly withdrawn from the solution and then rotated throughout their switch to the higher stage of the machine, to find a way to type a movie of uniform thickness. When they attain the rear of the machine, the bars are transferred again to the decrease level and pass by way of additional drying kilns until they attain the entrance of the machine. Here, the dried films are removed from the moulds and cut to the correct length, the 2 elements are joined collectively and the complete capsule is delivered from the machine. The lubricant used is a release help to allow the dried capsules to be removed from the mould pins without damage. Hypromellose options behave in an identical way to gelatin, besides that the velocity of gelling is slower and thus the machine output is lowered. The output per machine is more than 1 million capsules per day, relying on the scale: the smaller the capsule, the higher the output. The capsules now move through a series of sorting and checking processes, which could be both mechanical or electronic, to take away as many faulty ones as possible. The high quality levels are checked all through the process utilizing standard statistical sampling plans based mostly on military inspection standards. This is finished by an offset gravure roll printing process utilizing edible inks based on shellac. The info printed is often the product name or energy, a company name or emblem, or an identification code. The capsules are finally packed for shipment in moisture-proof liners, ideally heat-sealed aluminium foil baggage, in cardboard cartons. This is a vital factor to bear in mind throughout disintegration and dissolution testing. Because of this, most pharmacopoeias have set a limit of (37 � 2) �C for the media by which these tests are performed. Capsules created from hypromellose have a special solubility profile, being soluble at temperatures as low as 10 �C (Chiwele et al. Capsule filling Capsule sizes Hard capsules are made in a range of sizes; the standard industrial ones in use right now for human medicines vary in size from 0 to four (Table 33. To estimate the fill weight for a powder, the only way is to multiply the body volume by its tapped bulk density. The fill weight for liquids is calculated by multiplying the specific gravity of the liquid by the capsule body quantity multiplied by 0. The shape of the capsule has remained nearly unchanged since its invention greater than one hundred sixty years in the past, except for the development of the self-locking capsule through the 1960s, when computerized filling and packaging machines had been introduced. Filled capsules had been subjected to vibration throughout this process, causing some to come apart and spill their contents. To overcome this, fashionable capsule shells have a sequence of indentations on the within of the cap and on the external floor of the physique, which, when the capsule is closed after filling, type Table 33. The normal moisture content material specification for hard gelatin capsules is between 13% and 16% w/w. The moisture content material could be maintained throughout the appropriate specification by storing them in sealed containers at a good temperature. The manufacturer of the empty shells can be recognized from the indentations, which are specific to every one. Capsule shell filling Hard capsules could be filled with a large number of materials of different physicochemical properties. The limitations in the kinds of material that can be used to fill onerous capsules are shown in Table 33. The cause why such a spread of materials can be handled is the character of the capsule-filling course of. Metal fingers strike them in the center, and since the bodies have the smaller diameter, they rotate away from the path of influence. Next the capsules are sucked into pairs of bushings that trap the caps within the upper pair, because of their larger diameter, thus separating them from the our bodies. The caps are then repositioned over the bodies and metal fingers push the our bodies up into them to rejoin the two elements to the correct size so that the locking features of the capsule are engaged. Capsule-filling machines the identical set of primary operations is carried out whether capsules are being crammed on the bench for extemporaneous dishing out or on high-speed computerized machines for industrial merchandise. The major distinction between the many strategies obtainable is the best way during which the dose of material is measured into the capsule body. Empty capsules are fed into the holes, either manually or with a easy loading gadget. The bodies are locked of their plate by the use of a screw, and the caps of their plate are eliminated. Powder is placed onto this floor and is spread with a spatula so that it fills the our bodies. The uniformity of fill weight is very dependent on good flow properties of the powder.
Viagra plus 400 mg buy line
After each container has been shaken impotence genetic cheap viagra plus 400 mg overnight delivery, it should be emptied as fully as potential; the test is carried out on the person contents erectile dysfunction increases with age viagra plus 400 mg buy lowest price. As an instance, the British Pharmacopoeia defines an active substance as one the place a single dose of powder or granules incorporates an quantity of active substance less than 2 mg, or less than 2% of the whole mass of the single-dose preparation. Oral powders and granules provided in multidose containers should adjust to this test. The methods of manufacture of granules are mainly the identical no matter the destiny of the granules. However, within the context of manufacturing tablets, the mechanical properties of the granules and due to this fact the way in which by which they deform and bond are critical within the tableting course of. Pharmacopoeial checks the pharmacopoeial exams for assessing the standard of most powdered and granular dosage forms are very related. The function of those checks is indicated by its title; details of procedures and requirements can be discovered within the latest acceptable pharmacopeia. Single-dose oral powders ought to adjust to a pharmacopoeial test for uniformity of dosage units or, the place justified and licensed, with the checks for uniformity of content material and/or uniformity of mass. Pharmaceutical know-how of granule production Pharmaceutical granulation processes Granulation strategies may be divided into two varieties: wet methods, which use a liquid in the process, and dry methods, during which no liquid is used. Single-dose oral powders need to comply with a take a look at for uniformity of mass of single-dose preparations. The widespread sorts used are diluents, which are used to produce a unit dose weight of suitable measurement, and disintegrating brokers, which are added to assist the break-up of the granule when it reaches a liquid medium. An adhesive (also generally known as a binder) within the type of a dry powder may also be added, particularly if dry granulation is employed. Dry granulation In the dry strategies of granulation, the primary powder particles are aggregated at excessive stress. In both circumstances the intermediate product is damaged utilizing an appropriate milling approach to produce granular materials which is usually sieved to separate the specified size fraction. Organic solvents are used as an alternative choice to dry granulation when water-sensitive medicine are processed, or when a fast drying time is required. In the standard moist granulation method, the wet mass is pressured via a sieve to produce wet granules, which are then dried. A subsequent screening stage breaks agglomerates of granules and removes the fine materials, which could be recycled. Variations of this conventional methodology are depending on the tools used, but the general principle of preliminary particle aggregation utilizing a liquid remains in the entire processes. An various to the traditional moist granulation process is melt granulation whereby thermosetting polymers are used to kind the granules. Effect of the granulation method on granule construction the type and capability of granulating mixers considerably affect the work enter and time essential to produce a cohesive mass, sufficient liquid distribution and intragranular porosity of the granular mass. The technique and conditions of granulation affect intragranular pore structure by changing the degree of packing within particular person granules. It has been proven that precompacted granules, consisting of drug and binder particles, are held collectively by easy bonding shaped throughout consolidation. Granules prepared by wet massing include intact drug particles held collectively in a sponge-like matrix of binder. Fluidized-bed granules are much like granules ready by the wet-massing course of but possess higher porosity, and the granule floor is roofed by a film of binding agent. With spray-dried systems, the granules encompass spherical particles composed of an outer shell with an internal core of particles or air. Wet granulation (involving wet massing) Wet granulation includes the massing of a mix of dry major powder particles utilizing a granulating fluid. The granulating fluid accommodates a solvent that must be risky, in order that it might be eliminated by drying, and nontoxic. Typical suitable liquids include water, ethanol and 2-propanol both alone or together. The granulation liquid may be used alone or, more often, as a solvent containing a dissolved adhesive (also referred to as a binder or binding agent), which is used to ensure particle adhesion once the granule is dry. The disadvantages of water as a solvent are that it might adversely have an effect on drug stability, causing hydrolysis of prone merchandise, and it wants a longer drying time than natural solvents. This lengthy drying time increases the duration of the method and once more might have an result on chemical stability of the drug(s) due to the prolonged exposure to heat. The ones mentioned subsequent are those that are most related to pharmaceutical granulations. Adhesion and cohesion forces in motionless movies If adequate liquid is present in a powder to type a very thin, motionless layer, there might be an efficient decrease in interparticulate distance and a rise in contact area between the particles. The bond power between the particles might be increased because of this, because the van der Waals forces of attraction are proportional to the particle diameter and inversely proportional to the sq. of the gap of separation. This scenario will arise with adsorbed moisture and accounts for the cohesion of slightly damp powders. In dry granulation, nonetheless, the pressures used will enhance the contact area between the adsorbed layers and reduce the interparticulate distance, and this will contribute to the final granule energy. Thin, motionless layers may also be shaped by extremely viscous solutions of adhesives. The resulting bond strength will be larger than that produced by the cellular movies mentioned next. The use of starch mucilage in pharmaceutical granulations could produce this kind of film. Sufficient liquid is normally added to exceed that necessary for an immobile layer and this produces a cell film. At low moisture levels, termed the pendular state, the particles are held collectively by lens-shaped rings of liquid. These cause adhesion due to the surface rigidity forces of the liquid�air interface and the hydrostatic suction strain in the liquid bridge. When all the air has been displaced from between the particles, the capillary state is reached, and the particles are held by capillary suction at the liquid�air interface, which is now only at the granule surface. The funicular state represents an intermediate stage between the pendular and capillary states. Moist granule tensile strength increases by approximately three times from the pendular state to the capillary state. It may appear that the state of the powder bed depends upon the entire moisture content material of the wetted powders but the capillary state may also be reached by decreasing the separation of the particles. In the massing process during wet granulation, continued kneading/mixing of fabric initially within the pendular state will densify the moist mass, decreasing the pore volume occupied by air and finally producing the funicular or capillary state with out further liquid addition. In this state the energy of the droplet is dependent upon the surface tension of the liquid used.